AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2637-8876/014
1 Department of Pharmaceutical Microbiology, School of Pharmacy, Muhimbili University of Health and Allied Sciences, P.O. Box 65013,DaresSala
2 Department of Clinical Pharmacy and Pharmacology, School of Pharmacy, Muhimbili University of Health and Allied Sciences, P.O. Box 65013 Dares Salaam, Tanzania.
3 Department of Internal Medicine, Mloganzila, Muhimbili National Hospital, P.O. Box 65000, Dares Salaam, Tanzania.
*Corresponding Author: George M. Bwire, Department of Pharmaceutical Microbiology, Muhimbili University of Health and Allied Sciences, Tanzania. E-mail: gbwire@muhas.ac.tz
Citation: Faustine Tungaraza, Augustino S. Kahere, George M. Bwire, Doreen Mloka, Fatuma F. Felician, Liberata Mwita et all (2019). Immune reconstitution inflammatory syndrome (IRIS) during the first six months of receiving antiretroviral in HIV-infected individuals: a retrospective cohort study.
J. Immunology and Inflammation.3(1);DOI:10.31579/2637-8876/014
Copyright: © 2019 George M. Bwire. This is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 24 July 2019 | Accepted: 02 August 2019 | Published: 14 August 2019
Keywords: IRIS; muhimbili national hospital; HAART; prevalence; risk factors
Introduction: Immune reconstitution inflammatory syndrome (IRIS) is referred to as the flare up of an underlying, previously undiagnosed infection or the worsening of a previously treated infection soon after antiretroviral therapy (ART) is started. Information about the prevalence and associated risk factors for IRIS in resource-constrained countries like Tanzania where access to ART is increasing is scarce. Therefore, this study was conducted to determine the prevalence and risk factors associated with IRIS among patients attending care and treatment clinic at Muhimbili National Hospital (CTC-MNH).
Methods: A retrospective cohort study was conducted in patients receiving highly active antiretroviral therapy (HAART) who attended CTC-MNH between July 2016 and June 2018. Mann Whitney test was used to compare median CD4+ cells count and viral load at baseline and after 6 months of treatment. Associated factors were analysed using multi-logistic regression. Statistics were done using GraphPad Prism 7 software and a p-value < 0.05 was considered statistically significant.
Results: Of 318 patients, 8.5% encountered IRIS. Compared to baseline readings, there were significant increases in CD4+ cells (P < 0.0001) and decrease in viral load count (P < 0.0001). Patients who did not adhere to HAART were more likely to develop IRIS [Adjusted Odds Ratio (AOR) = 4.2, 95% CI; 1.14-15.60, P = 0.03].
Conclusion: This study found relatively low prevalence of IRIS as compared to those reported elsewhere. Moreover, poor adherence to HAART was found to be a risk factor for IRIS.
The introduction of highly active antiretroviral therapy (HAART) in human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) patients leads to dramatic reduction in plasma viral load, progression to AIDS, overall mortality [1] and provide partial restoration of overall immune function [2] . Furthermore, these immunological changes correlate with reduction in the frequency of opportunistic infections and prolong survival [3].
In the mid-1990s, clinicians noticed that certain patients deteriorated after starting HAART despite having decreasing HIV-1 RNA levels and raising CD4 cell counts [4]. In these patients, use of HAART resulted to a pathological inflammatory response to either previously treated infections or subclinical -opportunistic infections [5]. This inflammation could result in deleterious clinical outcomes known as immune reconstitution diseases (IRD) or immune reconstitution inflammatory syndrome (IRIS) [6]. Several infectious agents such as cytomegalovirus virus, Mycobacterium tuberculosis or Mycobacterium avium complex, and Cryptococcus neoformans have been associated with IRIS in HIV/AIDS patients starting HAART [4,7,8].
Although, information regarding IRIS prevalence and risk factors for developing IRIS and long term clinical outcomes is scarce, it is estimated that people living with HIV (PLHIV) and initiated with HAART have an IRIS prevalence ranging between 10-30% [5]. The variations in reported frequency are due to differences in case definitions, and more importantly differences in study populations with different risk profile and underlying burden of opportunistic infections [8]. It is expected that IRIS will be more common in low and middle-income countries, Tanzania in particular where access to antiretroviral therapy (ART) is increasing [9,10].
Therefore, the current study was conducted to determine the prevalence and risk factors associated with IRIS at Muhimbili National Hospital, care and treatment clinic.
Study design, area and subjects
A retrospective study was conducted in patients on HAART attending care and treatment clinic (CTC) at Muhimbili National Hospital (MNH) between July 2016 and June 2018. Since 2016 MNH-CTC adopted the use of both CD4+ cells counts and viral load as HIV/AIDS prognostic tests. Moreover, the implementations of diagnose and treat was still at infancy stage. MNH has a separate CTC with more than 18000 PLHIV on follow-up where about 500 PLHIV visit clinic per week. All patients received HAART for at least six months were eligible to participate in this study.
The sample size was calculated using the prevalence of 31.7% [5], where the marginal of error was set to be 5. Patients’ records at MNH records department were retrieved from the Jeeva HIV-care database (MNH, Tanzania). Information of patients enrolled between July 2016 and June 2018 were downloaded from the database then sampled randomly to attain the required sample size. The patient number was recorded then the respective file was reviewed so as to obtain the required information such as social demographics, lab investigations and treatment history. Information, which was not found in the patient’s file, was obtained from searching the specific information in the Jeeva HIV-care database.
Data collection
Development of case report forms/ checklist
Case report forms (CRFs) were developed to collect information including demographic, clinical, laboratory, drug administered and comorbidities. The CRFs were developed after comprehensive literature review of the studies, which described the association between IRIS and treatment outcomes [5,7,8]. Additionally, the CRFs were first validated and tested for answering the objectives of the study by selecting 10 patients’ files randomly from 318- sampled files.
Determination of IRIS
IRIS was determined after reviewing the patients’ files using criteria described elsewhere [6]. Briefly, new onset or worsening symptoms of an infection or inflammatory condition after start of HAART, progression of organ dysfunction or enlargement of pre- existing lesions after definite clinical improvement with pathogen-specific therapy prior to HAART and exclusion of treatment toxicity and new diagnoses and exaggerated inflammatory reaction i.e. severe fever or painful lesions were documented as IRIS. Two independent researchers reviewed the files, when the differences on documenting IRIS cases occurred, consensus were reached after consulting an experienced Clinician.
Documentation of adherence
Adherence was recorded as found in patients’ files. At MNH care and treatment clinic, adherence is commonly determined by patient’s self-reporting method. Based on that method more than 95% HAART take, coupled with clinical assessments. A Clinician assigned the status of “good adherence” (more than 95% HAART use in past seven and/or thirty days)” or “not good/ non-adherence” (less than 95% HAART use in the past seven and thirty days)” to the attended patient [9].
Definition of operational terms
Body mass-index (BMI): Calculated as the ratio of baseline weight (kg) and height (m2) of the patient obtained as documented in the file.
Immunological failure: This was the CD4+ cells count of less than 350 cells/mm3 for two consecutive measurements 6 months after initiation of antiretroviral [9].
Virological failure: This was the failure to suppress viral load to less than 1000 HIV-RNA copies/ml for two consecutive measurements within 6 months after initiation of antiretroviral [9].
Treatment failure: referred to either clinical failure occurrence / persistence of HIV related opportunistic infections or immunological and/or virological failures [9].
Data management and analysis
Data collected on CRFs were crosschecked for the completeness then entered manually in Microsoft excels spreadsheet followed by exporting them to GraphPad (Prism 7 software, USA) for analyzing social demographics and laboratory findings. Undocumented data were coded as missing information. Mean and percentage were used to summarize social demographic characteristics whereas Mann Whitney test was used to compare median CD4+ cells counts and viral load at baseline and six months after of receiving HAART. Associations between various factors with IRIS were analyzed using multi-logistic regression. Variables with p value less than 0.25 on univariate analysis were entered to multivariate analysis. Type 1 error for significance was 0.05.
Ethical consideration
Muhimbili University of Health and Allied Sciences (MUHAS) Research and Publication committee approved this study. (Ref. No. DA.282/298/01.C/). Permission to access patients’ files was requested from MNH Research and Publications Committee. Names and other personal details including patients’ hospital number were not disclosed for confidentiality purpose; thus, most information was coded and entered into the computer for statistical analysis.
Subjects recruitment flow chart
As of June 2018, a total of 18813 PLHIV were attending care and treatment clinic at MNH. Of 18813, 2.4% [number (n) = 457] were enrolled between July 2016 and June 2018 and 93% (n = 425) of the enrolled patients were initiated HAART, then 318 patients’ files of those receiving HAART were reviewed to determine the prevalence and risk factors for IRIS (Figure 1).
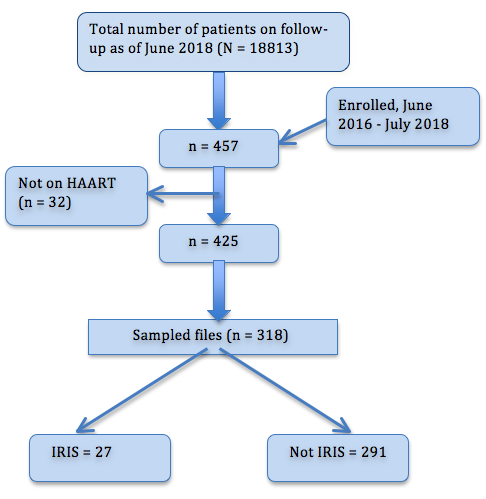
Figure 1: Subjects recruitment flow chart. A total of 318 files of patients attended clinic between July 2016 and June 2018 were sampled and reviewed.
Patients’ social demographic information
Overall 318 files of PLHIV attended CTC at MNH between July 2016 and June 2018 were reviewed. The patients’ age [±standard deviation (±SD)] was 38.3 years (15.7) with the mean BMI (±SD) of 24.5 kg/m2 (6.6). Majority of the participants (42.1%, n=102) were married while the number of females (55.7%, n=177) was higher. Most of the participants (35.7%, n=104) were at WHO AIDS first stage and 77.7% (n=247) were using Tenofovir based regimen (TDF). Fewer cases of co-infection (7.9%, n=25) were documented at the baseline where most (27.3%, n=6) of the participants were co-infected with pneumonia (Table 1).
Table 1: Participants’ social demographic information
Variable | Measurement | Value | |
Age (year) | Mean (±SD) | 38.3 (15.7) | |
Sex | Male, n (%) | 141 (44.3) | |
Female, n (%) | 177 (55.7) | ||
BMI (kg/m2) | Mean (±SD) | 24.5 (6.6) | |
Marital status | Single, n (%) | 99 (40.9) | |
Married, n (%) | 102 (42.1) | ||
Widow, n (%) | 26 (10.7) | ||
Divorced, n (%) | 15 (6.2) | ||
HAART regimen
| Tenofovir regimen, n (%) | 247 (77.7) | |
Zidovudine regimen, n (%) | 19 (6.0) | ||
Abacavir regimen, n (%) | 52 (16.4) | ||
WHO stage | I, n (%) | 104 (35.7) | |
II, n (%) | 66 (22.7) | ||
III, n (%) | 61 (21.0) | ||
IV, n (%) | 60 (20.6) | ||
Co-infection | Yes, n (%)
| Type of infection |
|
Herpes | 3 (13.6) | ||
TB | 4 (18.2) | ||
Pneumonia | 6 (27.3) | ||
Oral fungal infection | 4 (18.2) | ||
Skin fungal infection | 4 (18.2) | ||
Others | 1 (4.5) | ||
| Total Yes | 25 (7.9) | |
No, n (%) | 293 (92.1) | ||
Clinical characteristics for cohort of patients on HAART
The median CD4+ cell count [interquartile range (IQR)] at baseline and after six months were 237 cells/mm3 (126-438) and 391 cells/mm3 (224-647) respectively, P < 0.0001. The viral load count (log10copies/ml) at the baseline and after six months were 3.4 (2.5-4.7) and 2.4 (1.3-3.6) respectively, P < 0.0001. Among 318 studied patients the prevalence of IRIS was found to be 8.5%. The highest numbers of patients were reported to have good adherence to HAART use by 48.8% (n=120) (Table 2).
Table 2: Clinical characteristics for cohort of patients on HAART for at least 6 months
Variable | Value
| P value
| ||
Laboratory measurements
| CD4+ cell (cells/mm3) Median (IQR) | At baseline | 237 (126-438)
|
P < 0.0001 |
After 6 months | 391 (224-647)
| |||
Viral load (log10copies/ml) Median (IQR) | At baseline | 3.4 (2.5-4.7)
|
P < 0.0001 | |
After 6 months | 2.4 (1.3-3.6) | |||
IRIS | n (%) | 27 (8.5) |
| |
Adherence | Good, n (%) | 120 (48.8) |
| |
Not good, n (%) | 20 (6.3) | |||
Undocumented | 143 (45) | |||
Factors associated with IRIS
The study found no association between age, BMI, CD4+ cells count, viral load at baseline and WHO AIDS stage and IRIS (P>0.05). With regards to adherence, patients who did not adhere to HAART had four times chances to get IRIS [Adjusted Odds Ratio (OR) = 4.2, 95%CI; 1.14-15.60, P = 0.03] (Table
Table 3: Multi-logistic regression analysis of IRIS associated factors
Factor | Adjusted Odds Ratio (AOR) 95%CI | P value | |
Age (years) | 0.9 (0.9, 1.1) | P = 0.827 | |
BMI (kg/mm2) | 0.9 (0.77,1.2) | P = 0.808 | |
CD4+ cells count at baseline (cells/mm3) | 1 (0.99,1.0) | P = 0.736 | |
Viral load at baseline (copies/ml) | 1 (1,1) | P = 0.921 | |
Adherence
| Yes | 1.8 (0.74, 4.4) | P = 0.192 |
No | 4.2 (1.14, 15.60) | P = 0.031* | |
WHO AIDS stage | First | 0.45 (0.12,1.77) | P = 0.254 |
Second | 0.42 (0.1, 1.78) | P = 0.240 | |
Third | 0.5 (0.11, 2.3) | P = 0.376 | |
*Statistically significant | |||
The current HIV/AIDS management policy of Tanzania advocate diagnose and treat [9], however medical seeking behaviors in low and middle-income countries (LMICs) still relies on presentation of first disease symptoms [11]. Hence, an increased risk of HIV/AIDS and IRIS [8]. Moreover, increased use of HAART in LMICs, estimated to increase the incidences of IRIS [9]. Tanzania is among the countries where the use of ARV is increasing [9]. Therefore, a two years retrospective study (2016-2018) was conducted to determine the prevalence and factors associated with IRIS in patients attending care and treatment clinic at MNH. To the best of our knowledge this is the first study to report the prevalence and risk factors for IRIS at MNH.
The current study found a prevalence of IRIS to be 8.5%. This is a small percent when compared to the finding by Shelburne et al. 2005 [5] which reported the the prevalance of about 30%. Another study which was conducted in Perunian children documented a prevalance of 20% [12]. The differences between the studies may have been contributed by the different in study design [5] and difference in the study population [12]. Additionally the study participants in this study majority (35.7%) were at the first WHO AIDS stage and the mean age of participants in the current retrospective study was 38.3 years.
This study found a significant increase in CD4+ cells count and decrease in viral load six months after initiations of HAART. This concured to the study conducted in Rwanda which, reported similar clinical outcomes [13]. Findings from the current study may have been contributed by good adhrence reported to most of the patients (48.8%) and most of them being enrolled at WHO AIDS first stage (35.7%)
In determining the risks factors associated with IRIS, only non-adherence to HAART was associated with the risk of getting IRIS. Although the risks factors for getting IRIS are still unclear [5], but different factors such as low CD4+ counts, high viral load and presence of subclinical infections have been hypothesized to be responsible [6,8]. However the current study found no association between IRIS and these factors.
This study was limited by lack of standard definition of IRIS with objective biomarkers for diagnosis. Conforming to the national guidelines, publisher articles and having multiple reviewers to agree on suspected IRIS cases minimized the limitation. In addition, adherence was limited to patient’s memory but other parameters such as clinical assessment and laboratory investigations were included in assigning good or non-adherence.Moreover, because the design of the study was retrospective, some of the CD4 lymphocyte counts and HIV viral loads were not available at baseline, after HAART initiation and at the time of the IRIS event. Also, the number of eligible participants in the cohort was small to make firm conclusions about specific risk factors.
Conclusion
This study found relatively low prevalence of IRIS as compared to those reported elsewhere. Moreover, non-adherence to HAART was found to be a risk factor for IRIS. Recommendation, Clinicians should be aware of both the unmasked subclinical infections as well as the paradoxical recrudescence of successfully treated infections in HIV-infected patients shortly after initiating HAART. Future study on specific risk factors is recommended so that patients who are at highest risk of IRIS can be identified and treated promptly.
Abbreviations
AIDS: Acquired Immune Deficiency Syndrome; CD4: Cluster of Differentiation; CTC: Care and Treatment Clinic; HAART: Highly Active Antiretroviral Therapy; HIV: Human Immunodeficiency Virus; IRIS: Immune Reconstitution Inflammatory Syndrome; MNH: Muhimbili National hospital; PLHIV: People Living with HIV; RNA: Ribonucleic Acid; TB: Mycobacterium tuberculosis; WHO: World Health Organization.
Authors declare that there is no competing interest
We thank the MNH management for granting permission to access patients’ files on care and treatment clinic and collect their information. More importantly, we acknowledge the cooperation received from Clinicians, Nurses and Health Records Department particularly Mr. Geofrey Semu.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.
